Proven effective across all FDA-designated
co-primary endpoints1
Dyspareunia
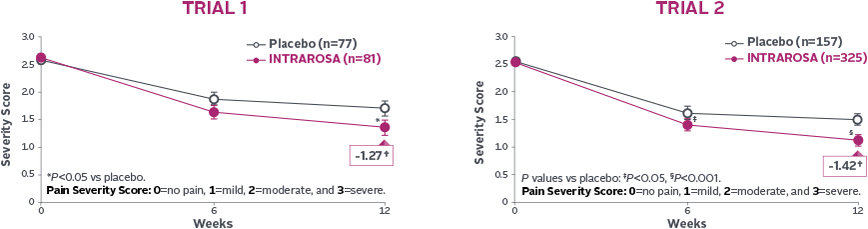
Statistically significant decrease in the mean severity of dyspareunia1-3
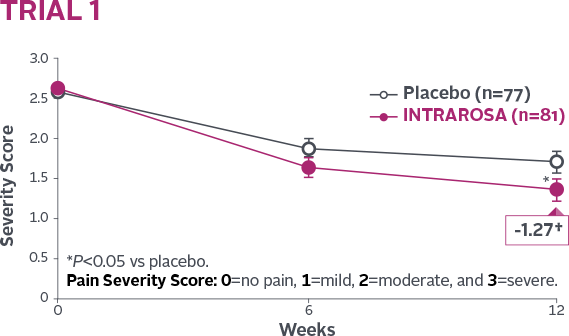
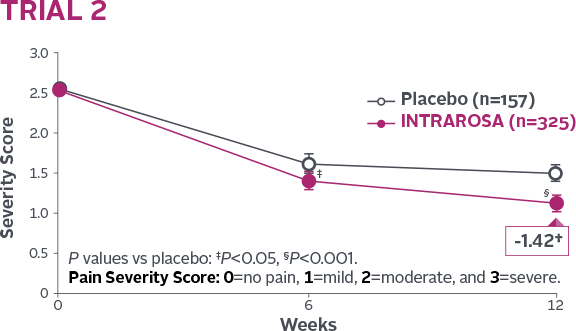
INTRAROSA lowered mean severity scores by 0.40 more than placebo in Trial 1 (P=0.013) and 0.35 more than placebo in Trial 2 (P=0.0002)1-3||
†Mean change from baseline in INTRAROSA group.
||Difference from placebo=INTRAROSA (Week 12 mean – Baseline mean) –
Placebo (Week 12 mean – Baseline mean).
STUDY DESIGN1,2
In two 12-week, randomized, double-blind, placebo-controlled phase 3 clinical trials, healthy postmenopausal women with moderate to severe dyspareunia, their most bothersome symptom being vulvovaginal atrophy (VVA), were evaluated. They also had ≤5% superficial cells on vaginal smear and a vaginal pH >5. From baseline to Week 12, all women were assessed for improvement in 4 co-primary efficacy endpoints: most bothersome moderate to severe symptom of dyspareunia, the percentage of vaginal superficial cells, the percentage of parabasal cells, and vaginal pH.
Superficial Cells
Statistically significant increase in % of superficial cells1-3
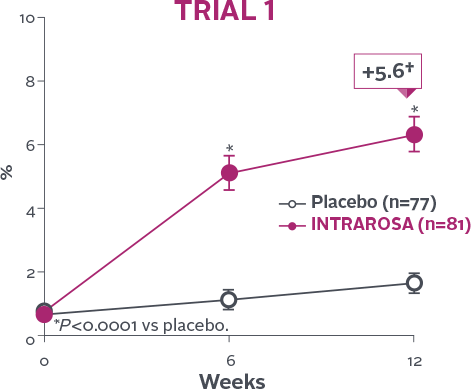
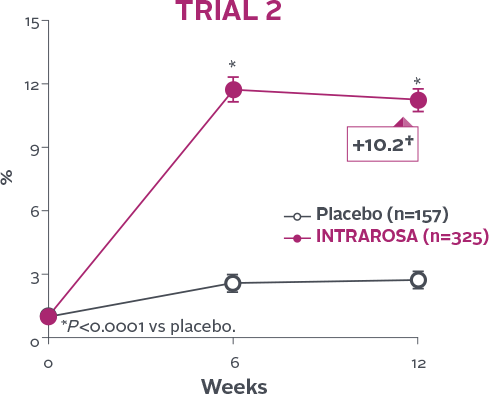
INTRAROSA increased the mean percentage of superficial cells by 4.71 percentage points over placebo in Trial 1 (P<0.0001)
and 8.46 percentage points over placebo in Trial 2 (P<0.0001)1-3‡
†Mean change from baseline in INTRAROSA group.
‡Difference from placebo=INTRAROSA (Week 12 mean – Baseline mean) –
Placebo (Week 12 mean – Baseline mean).
STUDY DESIGN1,2
In two 12-week, randomized, double-blind, placebo-controlled phase 3 clinical trials, healthy postmenopausal women with moderate to severe dyspareunia, their most bothersome symptom being vulvovaginal atrophy (VVA), were evaluated. They also had ≤5% superficial cells on vaginal smear and a vaginal pH >5. From baseline to Week 12, all women were assessed for improvement in 4 co-primary efficacy endpoints: most bothersome moderate to severe symptom of dyspareunia, the percentage of vaginal superficial cells, the percentage of parabasal cells, and vaginal pH.
Parabasal Cells
Statistically significant decrease in % of parabasal cells1-3
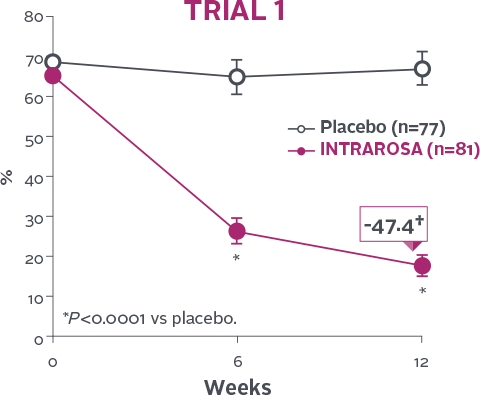
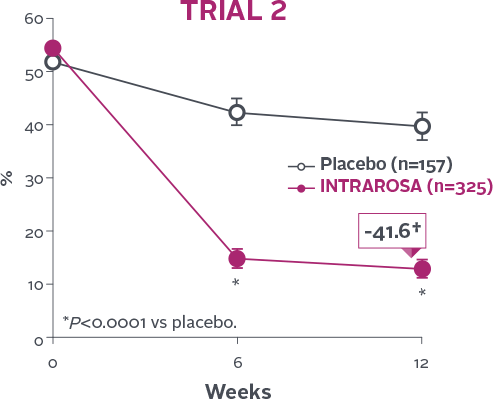
INTRAROSA decreased the mean percentage of parabasal cells by 45.77 percentage points over placebo in Trial 1
(P<0.0001) and 29.53 percentage points over placebo in Trial 2 (P<0.0001)1-3‡
†Mean change from baseline in INTRAROSA group.
‡Difference from placebo=INTRAROSA (Week 12 mean – Baseline mean) –
Placebo (Week 12 mean – Baseline mean).
STUDY DESIGN1,2
In two 12-week, randomized, double-blind, placebo-controlled phase 3 clinical trials, healthy postmenopausal women with moderate to severe dyspareunia, their most bothersome symptom being vulvovaginal atrophy (VVA), were evaluated. They also had ≤5% superficial cells on vaginal smear and a vaginal pH >5. From baseline to Week 12, all women were assessed for improvement in 4 co-primary efficacy endpoints: most bothersome moderate to severe symptom of dyspareunia, the percentage of vaginal superficial cells, the percentage of parabasal cells, and vaginal pH.
Vaginal pH
Statistically significant decrease in vaginal pH1-3
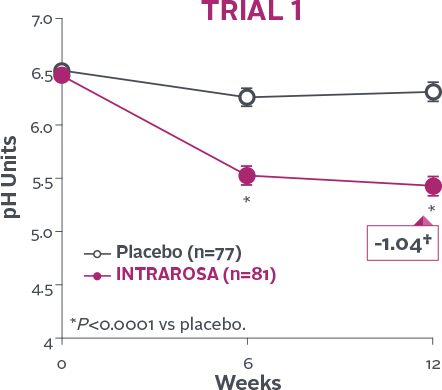
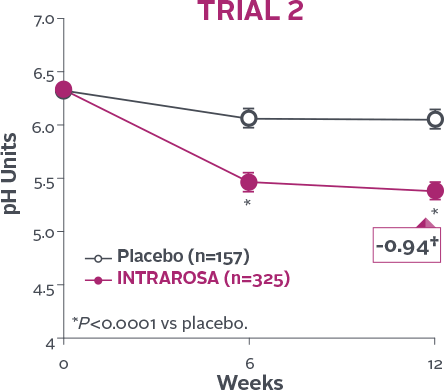
INTRAROSA lowered mean vaginal pH by 0.83 pH units more than placebo in Trial 1 (P<0.0001) and 0.67 pH units more than placebo in Trial 2 (P<0.0001)1-3‡
†Mean change from baseline in INTRAROSA group.
‡Difference from placebo=INTRAROSA (Week 12 mean – Baseline mean) –
Placebo (Week 12 mean – Baseline mean).
STUDY DESIGN1,2
In two 12-week, randomized, double-blind, placebo-controlled phase 3 clinical trials, healthy postmenopausal women with moderate to severe dyspareunia, their most bothersome symptom being vulvovaginal atrophy (VVA), were evaluated. They also had ≤5% superficial cells on vaginal smear and a vaginal pH >5. From baseline to Week 12, all women were assessed for improvement in 4 co-primary efficacy endpoints: most bothersome moderate to severe symptom of dyspareunia, the percentage of vaginal superficial cells, the percentage of parabasal cells, and vaginal pH.
Statistically significant decrease in the mean severity of dyspareunia1-3


INTRAROSA lowered mean severity scores by 0.40 more than placebo in Trial 1 (P=0.013) and 0.35 more than placebo in Trial 2 (P=0.0002)1-3||
†Mean change from baseline in INTRAROSA group.
||Difference from placebo=INTRAROSA (Week 12 mean – Baseline mean) –
Placebo (Week 12 mean – Baseline mean).
STUDY DESIGN1,2
In two 12-week, randomized, double-blind, placebo-controlled phase 3 clinical trials, healthy postmenopausal women with moderate to severe dyspareunia, their most bothersome symptom being vulvovaginal atrophy (VVA), were evaluated. They also had ≤5% superficial cells on vaginal smear and a vaginal pH >5. From baseline to Week 12, all women were assessed for improvement in 4 co-primary efficacy endpoints: most bothersome moderate to severe symptom of dyspareunia, the percentage of vaginal superficial cells, the percentage of parabasal cells, and vaginal pH.
Statistically significant increase in % of superficial cells1-3


INTRAROSA increased the mean percentage of superficial cells by 4.71 percentage points over placebo in Trial 1 (P<0.0001)
and 8.46 percentage points over placebo in Trial 2 (P<0.0001)1-3‡
†Mean change from baseline in INTRAROSA group.
‡Difference from placebo=INTRAROSA (Week 12 mean – Baseline mean) –
Placebo (Week 12 mean – Baseline mean).
STUDY DESIGN1,2
In two 12-week, randomized, double-blind, placebo-controlled phase 3 clinical trials, healthy postmenopausal women with moderate to severe dyspareunia, their most bothersome symptom being vulvovaginal atrophy (VVA), were evaluated. They also had ≤5% superficial cells on vaginal smear and a vaginal pH >5. From baseline to Week 12, all women were assessed for improvement in 4 co-primary efficacy endpoints: most bothersome moderate to severe symptom of dyspareunia, the percentage of vaginal superficial cells, the percentage of parabasal cells, and vaginal pH.
Statistically significant decrease in % of parabasal cells1-3


INTRAROSA decreased the mean percentage of parabasal cells by 45.77 percentage points over placebo in Trial 1
(P<0.0001) and 29.53 percentage points over placebo in Trial 2 (P<0.0001)1-3‡
†Mean change from baseline in INTRAROSA group.
‡Difference from placebo=INTRAROSA (Week 12 mean – Baseline mean) –
Placebo (Week 12 mean – Baseline mean).
STUDY DESIGN1,2
In two 12-week, randomized, double-blind, placebo-controlled phase 3 clinical trials, healthy postmenopausal women with moderate to severe dyspareunia, their most bothersome symptom being vulvovaginal atrophy (VVA), were evaluated. They also had ≤5% superficial cells on vaginal smear and a vaginal pH >5. From baseline to Week 12, all women were assessed for improvement in 4 co-primary efficacy endpoints: most bothersome moderate to severe symptom of dyspareunia, the percentage of vaginal superficial cells, the percentage of parabasal cells, and vaginal pH.
Statistically significant decrease in vaginal pH1-3


INTRAROSA lowered mean vaginal pH by 0.83 pH units more than placebo in Trial 1 (P<0.0001) and 0.67 pH units more than placebo in Trial 2 (P<0.0001)1-3‡
†Mean change from baseline in INTRAROSA group.
‡Difference from placebo=INTRAROSA (Week 12 mean – Baseline mean) –
Placebo (Week 12 mean – Baseline mean).
STUDY DESIGN1,2
In two 12-week, randomized, double-blind, placebo-controlled phase 3 clinical trials, healthy postmenopausal women with moderate to severe dyspareunia, their most bothersome symptom being vulvovaginal atrophy (VVA), were evaluated. They also had ≤5% superficial cells on vaginal smear and a vaginal pH >5. From baseline to Week 12, all women were assessed for improvement in 4 co-primary efficacy endpoints: most bothersome moderate to severe symptom of dyspareunia, the percentage of vaginal superficial cells, the percentage of parabasal cells, and vaginal pH.
Indication
INTRAROSA is a steroid indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause.
Important Safety Information
INTRAROSA is contraindicated in women with undiagnosed abnormal genital bleeding.
Estrogen is a metabolite of prasterone. Use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer. INTRAROSA has not been studied in women with a history of breast cancer.
In four 12-week randomized, placebo-controlled clinical trials, the most common adverse reaction with an incidence ≥2 percent was vaginal discharge. In one 52-week open-label clinical trial, the most common adverse reactions with an incidence ≥2 percent were vaginal discharge and abnormal Pap smear.
To report SUSPECTED ADVERSE REACTIONS, contact Millicent Pharma at 1-877-810-2101 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.
References: 1. Archer DF, Labrie F, Bouchard C, et al. Treatment of pain at sexual activity (dyspareunia) with intravaginal dehydroepiandrosterone (prasterone). Menopause. 2015;22(9):950-963. 2. Labrie F, Archer DF, Koltun W, et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016;23(3):243-256. 3. Intrarosa [package insert]. East Hanover, NJ: Millicent Pharma Limited; 2020.