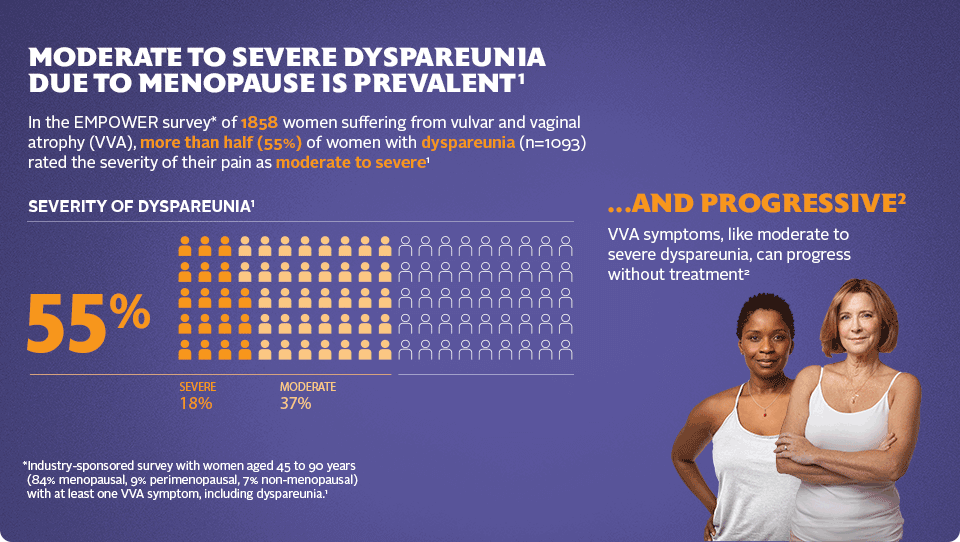
Moderate to Severe Dyspareunia due to Menopause is Prevalent1
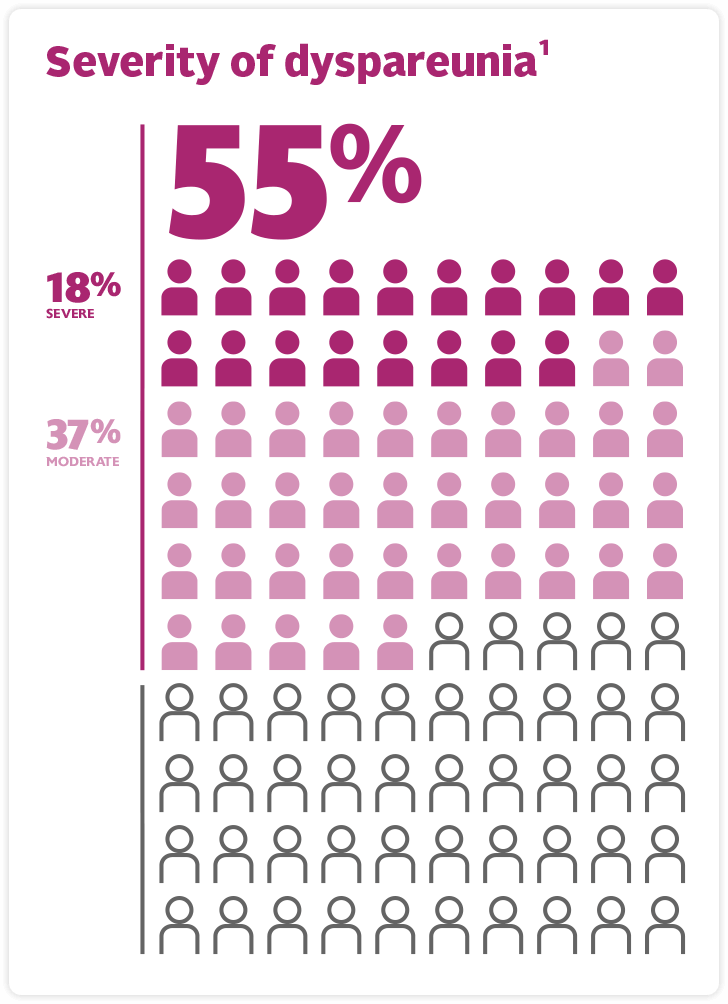
- Industry-sponsored survey with women aged 45 to 90 years (84% menopausal, 9% perimenopausal; 7% non-menopausal) with at least one VVA symptom, including dyspareunia.1
…and progressive2
Discomfort, but not enough dialogue…
Your patients with moderate to severe dyspareunia due to menopause may not bring it up. Proactively asking patients with VVA about their symptoms can be an important first step toward management.2Indication
INTRAROSA is a steroid indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause.
Important Safety Information
INTRAROSA is contraindicated in women with undiagnosed abnormal genital bleeding.
Estrogen is a metabolite of prasterone. Use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer. INTRAROSA has not been studied in women with a history of breast cancer.
In four 12-week randomized, placebo-controlled clinical trials, the most common adverse reaction with an incidence ≥2 percent was vaginal discharge. In one 52-week open-label clinical trial, the most common adverse reactions with an incidence ≥2 percent were vaginal discharge and abnormal Pap smear.
To report SUSPECTED ADVERSE REACTIONS, contact Millicent Pharma at 1-877-810-2101 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.
References: 1. Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women’s EMPOWER survey: identifying women’s perceptions on vulvar and vaginal atrophy and its treatment. J Sex Med. 2017;14:413-424. 2. Wysocki S, Kingsberg A, Krychman M. Management of vaginal atrophy: implications from the REVIVE Survey. Clin Med Insights Reprod Health. 2014;8:23-30.

