
Intrarosa is the FIRST AND ONLY FDA-APPROVED NON-ESTROGEN THERAPY WITH PRASTERONE1
Prasterone is a plant-derived version of endogenous DHEA2,3
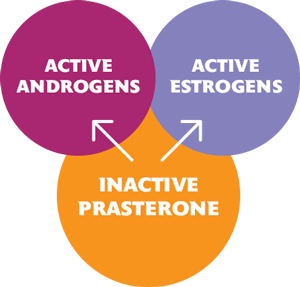
Prasterone steroidogenesis1
- Prasterone, an inactive steroid, is converted into biologically active androgens and estrogens. The mechanism of action of INTRAROSA is not fully established1
- Exogenous prasterone is metabolized in the same manner as endogenous prasterone1
- In humans, intracellular steroidogenic enzymes transform prasterone into the sex steroids – androgens and estrogens1
Indication
INTRAROSA is a steroid indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause.
Important Safety Information
INTRAROSA is contraindicated in women with undiagnosed abnormal genital bleeding.
Estrogen is a metabolite of prasterone. Use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer. INTRAROSA has not been studied in women with a history of breast cancer.
In four 12-week randomized, placebo-controlled clinical trials, the most common adverse reaction with an incidence ≥2 percent was vaginal discharge. In one 52-week open-label clinical trial, the most common adverse reactions with an incidence ≥2 percent were vaginal discharge and abnormal Pap smear.
To report SUSPECTED ADVERSE REACTIONS, contact Millicent Pharma at 1-877-810-2101 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.
References: 1. Intrarosa [package insert]. East Hanover, NJ: Millicent Pharma Limited; 2020. 2. Origin Statement. Veracruz, Mexico: Productos Químicos Naturales S.A. de C.V (Proquina). 2017. 3. Witepsol Origin Statement. Hamburg, DE: IOI Oleochemical. 2016.

