INTRAROSA PHARMACOKINETICS1
Post Day-7 Cmax
Cmax of prasterone, testosterone, and estradiol on Day 7 following daily administration of placebo or INTRAROSA (mean±SD)1*
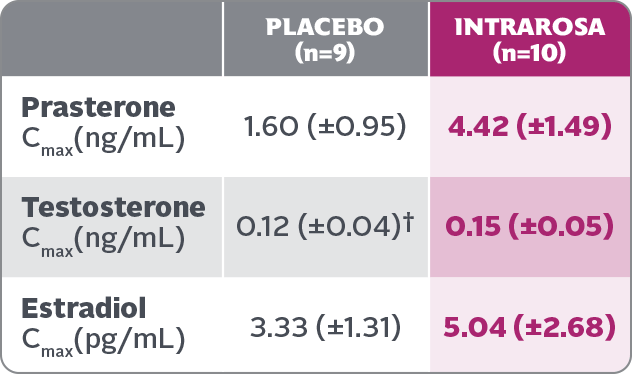
- Mean prasterone Cmax after 7 days was significantly higher in women treated with INTRAROSA after 7 days vs those treated with placebo. The Cmax of testosterone and estradiol were slightly higher
in women treated with INTRAROSA after 7 days vs those treated with placebo.
†n=8.
Post Day-7 Prasterone
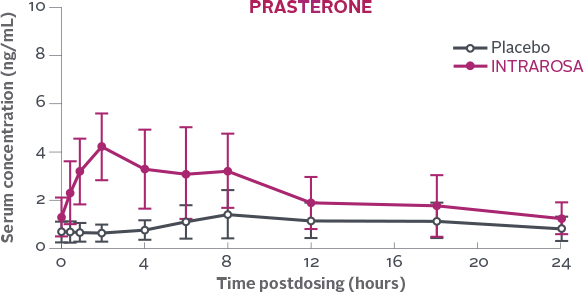
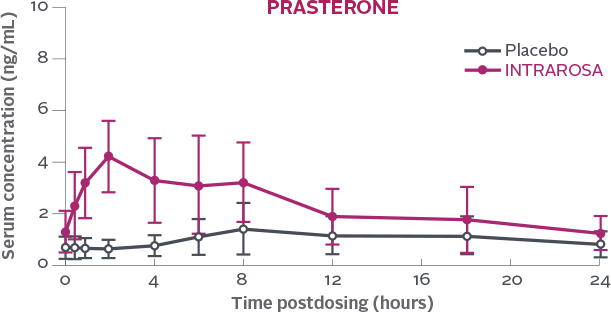
Serum concentration of prasterone measured over a 24h period on Day 7 following once-daily bedtime administration of placebo or INTRAROSA1
- In 2 primary efficacy trials, daily administration of an INTRAROSA vaginal insert for 12 weeks increased mean serum Ctrough of prasterone and its metabolites testosterone and estradiol by 47%, 21%,
and 19% from baseline, respectively
- This comparison based on Ctrough may underestimate the magnitude of increase in prasterone and its metabolites’ exposure because it does not take into account
the overall concentration-time profile following administration of INTRAROSA
Post Day-7 Testosterone
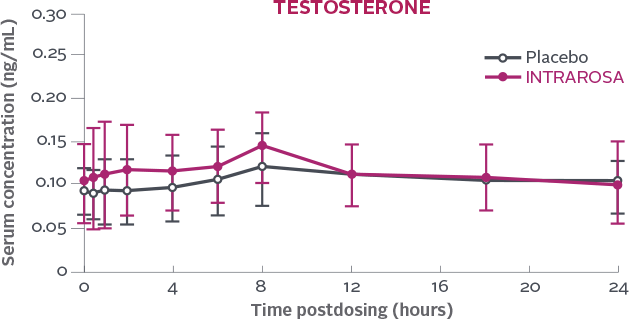
Serum concentration of testosterone measured over a 24h period on Day 7 following once-daily bedtime administration of placebo or INTRAROSA1
- In 2 primary efficacy trials, daily administration of an INTRAROSA vaginal insert for 12 weeks increased mean serum Ctrough of prasterone and its metabolites testosterone and estradiol by 47%, 21%,
and 19% from baseline, respectively
- This comparison based on Ctrough may underestimate the magnitude of increase in prasterone and its metabolites’ exposure because it does not take into account
the overall concentration-time profile following administration of INTRAROSA
Post Day-7 Estradiol (E2)
Serum concentration of estradiol measured over a 24h period on Day 7 following once-daily bedtime administration of placebo or INTRAROSA1
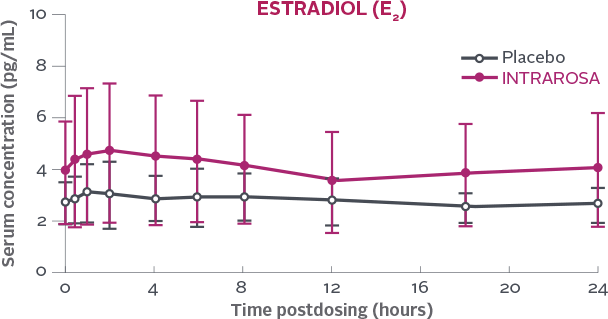
- In 2 primary efficacy trials, daily administration of an INTRAROSA vaginal insert for 12 weeks increased mean serum Ctrough of prasterone and its metabolites testosterone and estradiol by 47%, 21%,
and 19% from baseline, respectively
- This comparison based on Ctrough may underestimate the magnitude of increase in prasterone and its metabolites’ exposure because it does not take into account
the overall concentration-time profile following administration of INTRAROSA
Cmax of prasterone, testosterone, and estradiol on Day 7 following daily administration of placebo or INTRAROSA (mean±SD)1*

- Mean prasterone Cmax after 7 days was significantly higher in women treated with INTRAROSA after 7 days vs those treated with placebo. The Cmax of testosterone and estradiol were slightly higher in women treated with INTRAROSA after 7 days vs those treated with placebo.
†n=8.
Serum concentration of prasterone measured over a 24h period on Day 7 following once-daily bedtime administration of placebo or INTRAROSA1

- In 2 primary efficacy trials, daily administration of an INTRAROSA vaginal insert for 12 weeks increased mean serum Ctrough of prasterone and its metabolites testosterone and estradiol by 47%, 21%, and 19% from baseline, respectively
- This comparison based on Ctrough may underestimate the magnitude of increase in prasterone and its metabolites’ exposure because it does not take into account the overall concentration-time profile following administration of INTRAROSA
Serum concentration of testosterone measured over a 24h period on Day 7 following once-daily bedtime administration of placebo or INTRAROSA1

- In 2 primary efficacy trials, daily administration of an INTRAROSA vaginal insert for 12 weeks increased mean serum Ctrough of prasterone and its metabolites testosterone and estradiol by 47%, 21%, and 19% from baseline, respectively
- This comparison based on Ctrough may underestimate the magnitude of increase in prasterone and its metabolites’ exposure because it does not take into account the overall concentration-time profile following administration of INTRAROSA
Serum concentration of estradiol measured over a 24h period on Day 7 following once-daily bedtime administration of placebo or INTRAROSA1

- In 2 primary efficacy trials, daily administration of an INTRAROSA vaginal insert for 12 weeks increased mean serum Ctrough of prasterone and its metabolites testosterone and estradiol by 47%, 21%, and 19% from baseline, respectively
- This comparison based on Ctrough may underestimate the magnitude of increase in prasterone and its metabolites’ exposure because it does not take into account the overall concentration-time profile following administration of INTRAROSA
Indication
INTRAROSA is a steroid indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause.
Important Safety Information
INTRAROSA is contraindicated in women with undiagnosed abnormal genital bleeding.
Estrogen is a metabolite of prasterone. Use of exogenous estrogen is contraindicated in women with a known or suspected history of breast cancer. INTRAROSA has not been studied in women with a history of breast cancer.
In four 12-week randomized, placebo-controlled clinical trials, the most common adverse reaction with an incidence ≥2 percent was vaginal discharge. In one 52-week open-label clinical trial, the most common adverse reactions with an incidence ≥2 percent were vaginal discharge and abnormal Pap smear.
To report SUSPECTED ADVERSE REACTIONS, contact Millicent Pharma at 1-877-810-2101 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.
Reference: 1. Intrarosa [package insert]. East Hanover, NJ: Millicent Pharma Limited; 2020.